Abstract
Background Despite high response rates, durable responses are not achieved in most adults with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) patients (pts) receiving CD19 CAR T-cell therapy. We previously identified CD8+ T-cell responses to peptides mapped to the murine CD19-directed single chain variable fragment (scFv) of the CAR, potentially limiting CAR-T cell persistence and anti-tumor effects. We report the results of 23 B-ALL pts treated with a novel CAR T-cell therapy product using a fully human CD19-targeted scFv (JCAR021).
Methods Adult R/R B-ALL pts were enrolled between 2018 and 2022 on a phase I study investigating lymphodepletion (LD) with cyclophosphamide 300 mg/m2/d and fludarabine 30 mg/m2/d for 3 days followed by infusion of JCAR021 for R/R B-cell malignancies (NCT03103971). Pts were enrolled into 1 of 2 cohorts: high marrow burden ALL (HMB; > 5% blasts in bone marrow [BM] before LD; n=12); low marrow burden ALL (LMB; ≤ 5% blasts in BM before LD; n=11). The starting dose was 7x104 or 7x105 cells/kg for the HMB and LMB cohort, respectively. Responses were assessed per 2018 NCCN criteria 28 days after JCAR021 infusion. Measurable residual disease (MRD) was assessed using multiparameter flow cytometry. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were graded according to the Lee 2014 consensus criteria and the CTCAE v4.03 criteria, respectively. Median follow-up was 36 months.
Results Of 25 screened pts, 23 (92%) underwent leukapheresis, LD and JCAR021 infusion. Pts received JCAR021 at one of 5 dose levels (7x104/kg, n=3; 2x105/kg, n=4; 7x105/kg, n=8; 2x106/kg, n=7; 7x106/kg, n=1). Median preLD marrow blast percentage in HMB pts was 53% (range: 8-97). Median prior lines of therapy (excluding transplant) was 3 (range: 1-6); 15 (65%) pts had received a prior allogeneic hematopoietic cell transplant (allo-HCT). Thirteen (57%) had received blinatumomab; 12 (52%) had received inotuzumab ozogamicin. CRS and ICANS rates were 83% (grade ≥3, 0%) and 43% (grade ≥3, 30%), respectively. We observed a higher rate of CRS (92% versus 73%, respectively) and ICANS (50% versus 36%, respectively) in the HMB cohort compared to LMB, but comparable grade ≥3 CRS (0% in both cohorts) and ICANS (33% versus 27%, respectively). Dose-limiting toxicities were reported in two HMB pts treated with 7x105 JCAR021 cells/kg (refractory septic shock during grade 3 ICANS, n=1; prolonged grade 4 ICANS, n=1).
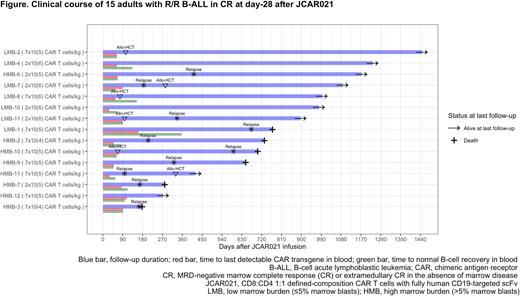
Twenty-two pts were evaluable for response assessment at day 28. In pts with evaluable BM disease (n=17), the MRD-negative CR rate was 82% (100% in pts treated with ≥7x105 cells/kg). In pts with measurable extramedullary disease (EMD; n=12), the overall response rate was 83% (CR, 33%). In CR (MRD-negative BM CR and/or PET-negative EMD CR) pts (n=15, 68%), the 36-month event-free survival (EFS) and overall survival (OS) were 30% and 55%, respectively. In all 23 infused pts, the estimated 36-month EFS and OS were 19% and 40%, respectively. Four of 18 pts (22%) in CR after treatment underwent consolidative allo-HCT. Of 5 CR pts still alive and leukemia-free at last follow-up, 2 underwent consolidative allo-HCT, while 3 did not receive additional therapies.
Despite receiving lower doses of JCAR021 (median: HMB, 2x105 CAR T cells/kg; LMB, 2x106 CAR T cells/kg), we measured higher peak CAR transgene expression in HMB compared to LMB pts (median, 31,774 versus 7,960 copies/µg DNA, respectively; p=0.012). Comparable CAR T-cell in vivo persistence was observed in CR pts in both HMB and LMB cohort (median time to last detectable CAR transgene in blood: 62 versus 63 days, respectively). Median time to B-cell recovery in the HMB and LMB group was 76 and 112 days, respectively. Loss of CAR transgene persistence and B-cell recovery were noted in all 10 CR pts who subsequently relapsed. In CR pts who subsequently relapsed, CD19 expression on B-ALL blasts was confirmed in all pts with available data (n=9).
Conclusion CD19 CAR T cells bearing a fully human scFv for R/R adult B-ALL elicited high rates of MRD-negative marrow and EM responses. Durable responses in the absence of consolidative allo-HCT were observed in a small subset of pts. All relapse events were CD19+ and occurred after loss of CAR T-cell persistence and B-cell recovery. Additional strategies are needed to prolong in vivo CAR T-cell persistence and improve outcomes of CD19 CAR T-cell therapy for adult R/R B-ALL.
Disclosures
Gauthier:Legend Biotech/Janssen: Consultancy; Kite Pharma: Consultancy; MorphoSys: Consultancy; Sobi: Research Funding; Juno Therapeutics, a Bristol Myers Squibb: Research Funding; Celgene, a Bristol Myers Squibb: Research Funding; Angiocrine Bioscience: Research Funding; Multerra Bio: Consultancy. Fiorenza:Bristol Myers Squibb: Patents & Royalties, Research Funding. Kimble:Juno Therapeutics, a BMS company: Research Funding. Vinaud Hirayama:Novartis: Honoraria; Juno Therapeutics, a BMS Company: Research Funding; Nektar Therapeutics: Research Funding; Bristol Myers Squibb: Honoraria. Shadman:Fate Therapeutics: Consultancy; MEI Pharma: Consultancy; Epi Lilly: Consultancy; Pharmacyclics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Sound Biologics: Consultancy; Regeneron: Consultancy; Genentech: Consultancy, Research Funding; Mustang Bio: Consultancy, Research Funding; Adaptimmune: Consultancy; Beigene: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Morphosys/Incyte: Consultancy, Research Funding; Epizyme: Consultancy; TG Therapeutics: Consultancy, Research Funding; Innate Pharma: Consultancy; Adaptive Biotechnologies: Consultancy; Merck: Consultancy; Kite Pharma: Consultancy; Abbvie: Consultancy, Research Funding; Atara Biotherapeutic: Consultancy, Research Funding; Celgene, a BMS Company: Research Funding; Gilead: Research Funding; Sunesis: Research Funding; Genmab: Research Funding. Cassaday:Amgen: Consultancy, Honoraria, Research Funding; Kite Pharma: Research Funding; Merck: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Vanda Pharmaceuticals: Research Funding; Seattle Genetics: Other: Spouse is employed by and owns stocks in Seattle Genetics. Riddell:Juno Therapeutics, a BMS Company: Current equity holder in publicly-traded company, Patents & Royalties; Nohla: Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Lyell Immunopharma: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees, Other: Founder, Patents & Royalties. Maloney:Novartis: Consultancy, Honoraria; Mustang Bio: Consultancy, Honoraria; Legend Biotech: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; MorphoSys/Incyte: Consultancy, Honoraria; Gilead Sciences: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite Pharma, a Gilead Company: Consultancy, Honoraria, Research Funding; Celgene, a BMS Company: Consultancy, Honoraria, Research Funding; Juno Therapeutics, a BMS Company: Consultancy, Honoraria, Patents & Royalties, Research Funding; A2 Biotherapeutics: Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees; Umoja: Consultancy, Honoraria; Navan Technologies: Current holder of stock options in a privately-held company, Honoraria, Membership on an entity's Board of Directors or advisory committees. Turtle:Allogene: Membership on an entity's Board of Directors or advisory committees; Eureka Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Kite Pharma, a Gilead Company: Membership on an entity's Board of Directors or advisory committees; Expert Connect: Consultancy; Caribou Bioscience: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Myeloid Therapeutics: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Decheng Capital: Consultancy, Membership on an entity's Board of Directors or advisory committees; Century Therapeutics: Membership on an entity's Board of Directors or advisory committees; T-CURX: Membership on an entity's Board of Directors or advisory committees; Arsenal Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Precision Bioscience: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Juno Therapeutics, a BMS Company: Patents & Royalties, Research Funding; Prescient Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal